Research directions
Early events in virus infection
Viruses have adapted to use many different cellular molecules during their replication. To initiate infection, viruses first need to stably attach to a suitable target cell. While some viruses directly fuse with the plasma membrane others such as influenza A virus initiate cellular signaling to trigger endocytosis. Since viruses are submicrometer entities, these processes occur at the nanometer scale.
Our research aims to investigate how viruses interact with their target cells and initiate cellular signaling to induce changes such as endocytosis as required for virus entry.
Virus-cell interaction
Many viruses use cellular glycans for cell attachment. Protein-glycan interactions are typically of low affinity and thus require some degree of multivalency to stably attach the virus to the cell surface. Yet, if the interaction would be too strong the virus could be immobilized, which would be disadvantageous.
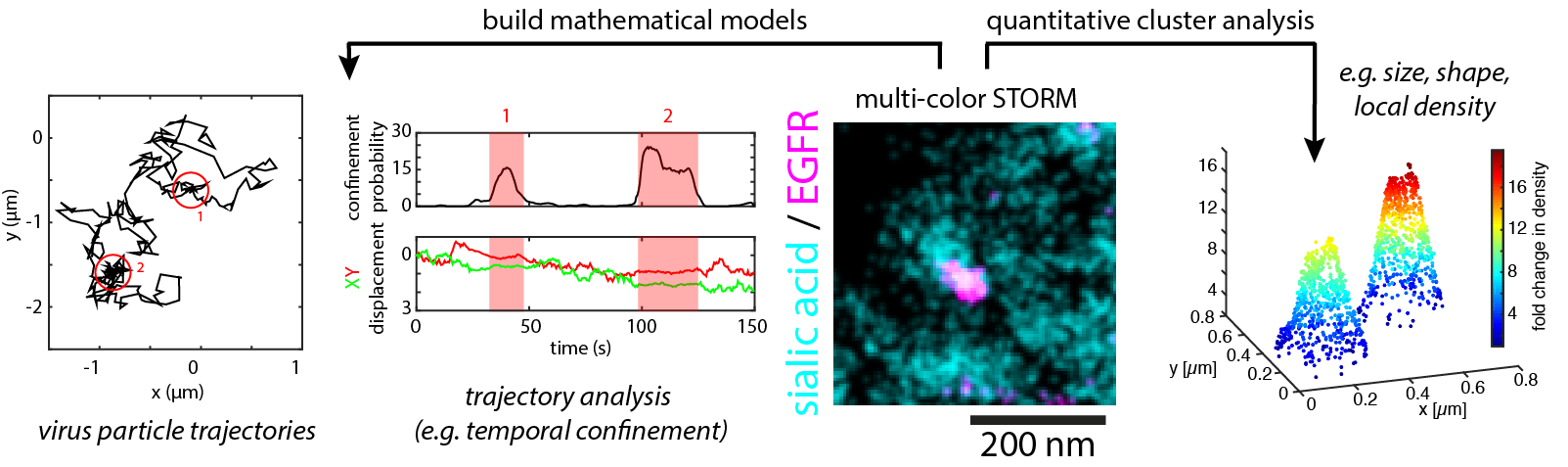
We study this predicament viruses are facing in their main goal to form a stable interaction with the cell surface but at the same time retaining flexibility to explore the cell surface. To target this goal we use biophysical techniques to study virus binding to live cells and single-particle tracking to follow individual viruses on their journey of infecting a cell.
Key publications: Broich et al., Nat Commun, 2025; Osman et al., Nat Commun, 2025; Herrmann & Sieben, Integr. Biology, 2015; Sieben et al., PNAS, 2014;
Plasma membrane organization and dynamics
Virus-cell interaction begins at the cellular plasma membrane. We use a number of different microscopy modalities with a focus on super-resolution techniques to investigate the organization and dynamics of individual cellular molecules involved in virus-cell interaction at the scale of the virus-cell interface (< 100 nm).
Using mainly techniques summarised as single-molecule localization microscopy (SMLM), our goal is to unravel the nanoscale cartography of the plasma membrane and in parallel study the dynamics of individual molecules involved in virus-cell interaction.
Key publications: Sieben et al., PLoS Pathogens, 2020; Köhler et al., Annual Reviews of Virology, 2020
Quantitative imaging
Imaging the virus-cell interface requires advanced microscopy techniques. We focus on SMLM techniques and apply a number of analysis schemes to extract quantitative information such as shape and density of nanostructures or estimate molecule numbers.
We further develop analysis procedures for high-throughput SMLM data. With a focus on particle datasets, our aim is to obtain high-resolution three-dimensional information of molecular complexes using particle averaging and 3D reconstruction.
Key publications: Degen et al., Nature, 2023; Glück et al., iScience, 2023; Sieben et al., Nat Methods, 2018; Prouteau et al., Nature, 2019
Current projects
COMBINE - Towards Improved Pandemic Preparedness
Advancing Our Understanding of Virus Cell Entry Using Marburg virus as a Model
The new EU project COMBINE (“Comparative Signature of Marburg Virus Cell Activation as a Blueprint for the Identification of Antiviral Targets against Newly Emerging Viruses”) acknowledges that understanding how viruses infiltrate host cells is crucial to combating emerging infectious diseases.
Please visit our website for more information.
Cell biology of respiratory virus infection.
How do viruses interact with their host cells? What signals are required and how are they interpreted by the cell? Our group studies the host-pathogen interaction of respiratory RNA viruses such as influenza A virus, respiratory syncytial virus (RSV), and SARS-CoV2. Our goal is to investigate how viruses use and alter cellular structures for cell entry and assembly, to understand how they exploit and control plasma membrane signaling, and whether these mechanisms can be applied to inhibit virus infection.
Virus-cortex interaction
Following virus-receptor activation, some viruses enter the cell by endocytosis and during this step need to overcome the cortical actin cytoskeleton. The cell cortex, a thin network of actin filaments bound to the plasma membrane is responsible for the cell’s mechanical stability and critically involved in morphogenesis. Cortical actin filament assembly is driven by F-actin nucleators, such as formins and the Arp2/3 complex. Our goal is to dissect the role and regulation of the cortical actin cytoskeleton during respiratory virus infection.
Molecular basis for host-cell adaptation and pathogenicity of zoonotic RNA viruses.
Understanding infectious diseases in animals and humans is of utmost importance to be prepared for an emerging and potentially zoonotic virus. Influenza A and orthohantaviruses are good examples of the tight relationship between the human population and the animal reservoir. We are using animal reservoir and human tissue culture systems to perform differential infection studies and identify host cell factors critically involved in virus infection and adaptation.